East African Sleeping Sickness Affect on the Angel Again War
- Review
- Open Access
- Published:
Civil disharmonize and sleeping sickness in Africa in general and Uganda in item
Disharmonize and Wellness book 1, Article number:6 (2007) Cite this commodity
Abstruse
Conflict and state of war have long been recognized equally determinants of infectious disease run a risk. Re-emergence of epidemic sleeping sickness in sub-Saharan Africa since the 1970s has coincided with all-encompassing civil conflict in affected regions. Sleeping sickness incidence has placed increasing pressure on the health resources of countries already burdened by malaria, HIV/AIDS, and tuberculosis. In areas of Sudan, the Democratic Republic of the congo, and Angola, sleeping sickness occurs in epidemic proportions, and is the first or second greatest cause of mortality in some areas, ahead of HIV/AIDS. In Uganda, at that place is evidence of increasing spread and institution of new foci in central districts. Conflict is an important determinant of sleeping sickness outbreaks, and has contributed to disease resurgence. This paper presents a review and characterization of the processes by which disharmonize has contributed to the occurrence of sleeping sickness in Africa. Conflict contributes to disease risk by affecting the transmission potential of sleeping sickness via economic impacts, degradation of health systems and services, internal displacement of populations, regional insecurity, and reduced access for humanitarian support. Particular focus is given to the case of sleeping sickness in south-eastern Uganda, where incidence increase is expected to continue. Disease intervention is constrained in regions with loftier insecurity; in these areas, political stabilization, localized deployment of health resource, increased administrative integration and national capacity are required to mitigate incidence. Conflict-related variables should exist explicitly integrated into take chances mapping and prioritization of targeted sleeping sickness enquiry and mitigation initiatives.
Background
Sleeping sickness re-emerged in Republic of uganda in the 1970s, and continues to pose a public health and economic brunt [one–3]. Similar re-emergence has been reported across sub-Saharan Africa since the 1970s, including outbreaks in the Democratic republic of the congo (DRC), Sudan [4], and Republic of angola [5]. In many cases, sleeping sickness outbreaks have coincided with periods of civil conflict and instability in affected countries and regions. Conflict in this context refers to the occurrence of civil war, rebel insurgency, violent governance, political or armed services oppression of populations, and military combat. These temporal associations are non purely spurious; patterns and processes related to conflict have been identified as determinants of sleeping sickness incidence and outbreaks [4, 5]. An improved understanding of the specific processes linking conflict to sleeping sickness incidence tin guide geographical predictions of affliction risk and optimization of intervention resources. This paper provides a review and characterization of the processes by which conflict has contributed to the occurrence of sleeping sickness outbreaks across sub-Saharan Africa, with a focus on due south-eastern Uganda (Figure 1).
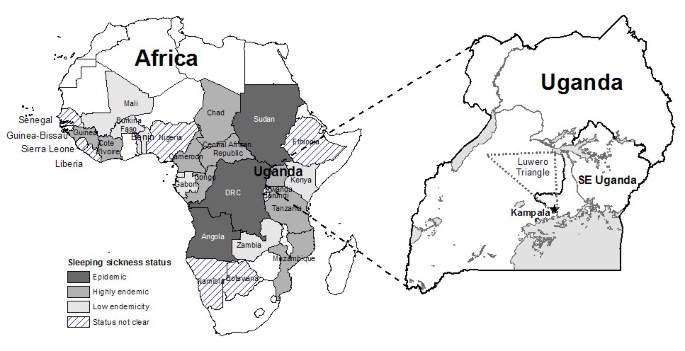
Distribution of sleeping sickness in Africa and map of Uganda showing the example written report area. The approximate location of the 'Luwero Triangle' is shown; this is where much of the conflict and violence was concentrated during Uganda's civil war (1979–86). Sleeping sickness condition data from WHO (2001).
Sleeping sickness: epidemiology, geographical distribution, and re-emergence
Sleeping sickness is the name used to describe the homo grade of African trypanosomiasis (Trypanosoma spp.), a protozoan parasitic disease affecting humans, livestock, and a large number of sylvatic species in much of sub-Saharan Africa (Figure 1). Transmitted by the tsetse fly vector (Glossina spp.), trypanosomiasis represents an important public health and economical burden in sub-Saharan Africa [6–8]. Sleeping sickness is characterized past highly variable and non-specific symptoms in its early stages [9], which are oft mis-diagnosed as malaria [x]. Late stage sleeping sickness includes body weakness, progressive emaciation, slurred speech, mental confusion, and coma leading to decease in all untreated cases [9]. There are two sub-species of human-infectious trypanosomes, including T. b. gambiense, which causes a more chronic disease, and is dominant in Western Africa, and T. b. rhodesiense, which causes more than acute illness, and is generally found in E and Southern Africa, e of the Rift Valley [xi]. The two forms of affliction have different environmental, pathology, and epidemiology. T. b. rhodesiense progresses from early non-specific symptoms to infection of the central nervous system and death within months, while T. b gambiense typically follows a chronic clinical form progressing over several years.
Sleeping sickness handling is expensive, complicated, and can exist unsafe for the patient [12]. Electric current drugs are in scarce or uncertain supply, and there is limited optimism with respect to forthcoming drugs entering the market, specially for the treatment of late-phase T. b. rhodesiense [12–17]. The dominant treatment for belatedly-stage sleeping sickness that involves the primal nervous system is melarsoprol, an organoarsenic compound with high toxicity and varying rates of treatment failure [12, 18]. Active surveillance and case treatment have been found to be extremely constructive in reducing disease manual, particularly for T. b. gambiense [12, 18], which is generally confined to a human-fly-human being cycle [xix]. T. b. rhodesiense transmission to humans is influenced by prevalence of the parasite in the brute reservoir; in eastward Africa, livestock correspond an of import reservoir for disease, and command of livestock infection and tsetse populations are important for reducing transmission to humans [12, 19].
Sleeping sickness was first identified and characterized in Africa in the last few years of the 19th century, a period that coincided with widespread and severe epidemics of the illness in Republic of kenya, Tanzania, Republic of uganda, Nigeria, and the Autonomous Republic of the congo (DRC). These epidemics have been associated with social and ecology disruptions during colonial assistants [11, 20], as well equally livestock restocking post-obit an 1889–1892 rinderpest epidemic [21]. The disease was generally brought under control by the 1960s in much of Africa only has re-emerged in many countries since the 1970s [8]. The re-emergence has been attributed to mail-independence political turbulence, unstable governments, express public wellness resources, and re-allocation of domestic and international funding towards malaria, HIV/AIDs, and tuberculosis.
In Republic of uganda, a large epidemic of T. b. rhodesiense began in 1976 in the south-east of the country (Effigy 1). Between 1976 and the decline of the epidemic in the mid-1990s, over 40,000 cases were reported in this region. Given estimates of significant under-reporting due to passive surveillance and diagnostic difficulties, the actual number of cases may accept been x times college, with all unreported and untreated cases assumed to be fatal [3, 22, 23]. Though incidence declined in the 1990s, the disease continues to spread into new regions. Recent research suggests that central Ugandan districts may be at specially loftier hazard of infection and increased incidence [24]. These reports are particularly pertinent given that Uganda represents the boundary between the ranges of the two sub-species of sleeping sickness. While T. b. rhodesiense continues to spread from its traditional focus in the south and east, cases of T. b. gambiense continue to exist recorded in north-western Uganda in the West Nile Region. These foci are currently separated past fewer than 200 kilometers, much of which is inhabited by tsetse flies. The two diseases differ widely in their treatment and command: it is very difficult to distinguish the two sub-species clinically [25]; a seriological card agglutination examination (CATT) is commonly used for T. b. gambiense diagnosis, just is inappropriate for T. b. rhodesience – the examination would non be constructive in the presence of both diseases [26]; drug handling regimes, which are expensive and can be unsafe, differ depending on the sub-species of infection [26]; approaches to control, such as focus on vector eradication, livestock treatment, or agile livestock or human surveillance, will be more than or less appropriate based on sub-species [25, 26]; there may exist potential for exchange of genetic material and increased drug resistance where the sub-species overlap [26, 27]; cost-effective and successful control of sleeping sickness would go extremely difficult to achieve where both species were present. The potential for overlap of the two illness foci is therefore a considerable economical and public health business concern [2].
Similar re-emergent outbreaks and incidence have been recorded in other countries (Figure ane). Angola experienced outbreaks in the late 1800s and early on 1900s. By 1974, the year earlier Angola's independence, nevertheless, only iii new cases of sleeping sickness were recorded [v]. Sleeping sickness re-emerged in Angola during a prolonged civil war post-obit the country's independence in 1975 [xix]. Although a peace agreement was signed in 2002, Republic of angola's infrastructure and political state of affairs remain highly unstable; the state is classified every bit 'epidemic' for sleeping sickness by the Earth Wellness Organization (WHO), forth with the Congo-kinshasa (DRC) and southern Sudan [28]. In Sudan, illness resurgence in the late 1970s was largely controlled by a Belgian-Sudanese trypanosomiasis handling and control initiative [18]. Civil war in the 1980s and 1990s, however, atomic number 82 to collapse of the command program, and by 1997, sleeping sickness had re-emerged in Sudan with prevalence rates as high as 19% in s-western communities bordering the Congo-kinshasa [18]. Re-emergence of disease and new epidemics were reported in the DRC in the 1970s and 1980s [29]. The DRC continues to experience Africa's highest burden of affliction from sleeping sickness; in 1994, 72% prevalence was reported in 1 village, Kimbanzi [xix]. The DRC represents an important source of infection for neighbouring countries. According to the WHO [28], Angola and southern Sudan have also reported loftier prevalences: between twenty% and 50% in some communities. In several areas, sleeping sickness is the first or second greatest cause of bloodshed, ahead of HIV/AIDS [19]. Currently, the DRC, Angola, and Sudan remain the countries about affected by sleeping sickness.
Countries considered to be 'highly endemic', with sleeping sickness re-emergence and expected incidence increase include Cameroon, Uganda, Central African Republic, Chad, Congo, Côte d'Ivoire, Republic of guinea, Mozambique, and Tanzania [28]. Incidence and prevalence are highly focal. In the Central African Republic, for example, villages adjoining Sudan have reported epidemic levels of disease [18]. Desultory or depression endemic levels of sleeping sickness take been reported in Benin, Burkina Faso, Equatorial Guinea, Gabon, Kenya, Republic of mali, Togo and Zambia. Unreported foci are likely to exist in additional countries, including Africa's almost populated nation, Nigeria [30]. While few sleeping sickness cases have been reported in Kenya since the late 1960s and early 1970s, cattle infection remains an economic burden and in that location is ongoing incidence of disease across the border in south-eastern Uganda.
Disharmonize, communicable diseases, and sleeping sickness
Disharmonize and war take long been recognized as determinants of infectious disease risk [31, 32]. Increased travel, trade, and inter-regional conflict have played a key role in the introduction and spread of diseases between civilisations and continents [33–35]. The influenza pandemic precipitated by World War I is a well-recognized instance of a illness epidemic emerging from disharmonize weather condition; deaths due to influenza exceeded deaths in battle [36]. Recent inquiry in the Democratic republic of the congo (DRC) found that elevated mortality during the recent civil war was closely associated with violence and that about deaths were due to malnutrition or infectious disease [37]. On-going armed conflict has hampered the international polio eradication campaign, peculiarly in Somalia and Afghanistan, areas of persistent polio infection and high insecurity [38, 39]. Outbreaks and increased disease incidence have been attributed to a range of factors associated with conflict [xl], including decreased hygiene, dietary deficiencies, reject of wellness services, travel insecurity, reduced access of humanitarian support, reduced veterinary and zoonoses command, and internal displacement of populations into marginal areas. The associations between conflict and communicable diseases are especially prevalent in Africa, where there remain many foci of ongoing civil disharmonize, and where infectious diseases remain important contributors to national mortality [41–43].
The sensitivity of sleeping sickness incidence to disharmonize-related processes is associated with the low transmission potential of the disease. A general model of the disease was adult by Rogers [44]. While developed for creature trypanosomiasis, an adapted and simplified equation can provide useful insights into the parameters affected the transmission potential of sleeping sickness; here we adapt the model for 1 tsetse species, one trypanosome species, and 1 host species – in this case nosotros are interested in man disease, simply animals are also important reservoirs of infection, specially in the instance of T. b. rhodesiense. The reproductive rate (R0) tin be estimated using the following general equation [44–46]:
R0 = α two mbce -u T/ur (Eq. 1)
Where:
R 0 Bones reproductive ratio
α Daily bitter rate of flies on humans (or beast reservoirs)
yard Ratio of tsetse flies to humans (or animals)
b Probability of a fly becoming infected from an infected person (or beast)
c Probability of a person (or animal) condign infected from an infected fly
1/u Life expectancy of tsetse flies (days)
T Incubation period in tsetse flies (days)
one/r Duration of infection in a person (or beast) (days)
R0 reflects the number of additional cases that a unmarried case is expected to generate and therefore represents the transmission potential of a pathogen; transmission will occur and epidemics tin result when R0 > i. The values of the parameters vary based on the species of host, parasite and vector of interest, as well equally the context of the area and state of affairs. In this example, we presume that the values of b, c, and T are constant and unrelated to political or social events. The remaining parameters, m, α, ane/u, and i/r, all the same, could vary due to external influences.
In humans, the R0 value is generally beneath ane and transmission cannot sustain itself; T. b. rhodesiense is mainly a zoonosis, with disease occurring commonly in cattle populations. In humans, periods of relatively low-level or undetected disease are punctuated past periods of astringent outbreak [27]. Sleeping sickness, particularly T. b. rhodesiense, is characterized by the occurrence of singled-out epidemics whose temporal occurrence has been consistently observed to parallel or lag periods of conflict in affected areas [24, 47]. This epidemic pattern is a role of the low transmission potential of sleeping sickness compared to other infectious diseases; one case of sleeping sickness will exist likely to result in farther cases only nether item circumstances strongly favouring transmission [8]. Transmission can be enhanced by increasing the equation parameters (Eq. 1) [8]; when sufficiently stiff or prolonged, an outbreak is triggered. Conflict-related processes, therefore, can influence sleeping sickness incidence by affecting the parameters in the model (1000, α, one/u, or 1/r); these tin include a range of ecological, social, and biophysical determinants [1]. Impacts on the biting charge per unit of flies (α), such as increased exposure of people to infested areas, will be particularly important as this parameter exponentially affects the value of R0. Similarly, atmospheric condition affecting the lifespan of the fly (ane/u), such equally vector control measures, will exist important as this parameter appears twice in the equation.
The case of south-eastern Uganda
Figure ii shows a time-line of sleeping sickness incidence in south-eastern Uganda betwixt 1900 and 2000. Equally seen in this figure, the epidemic of 1900–1920 coincides with institution of colonial rule in Republic of uganda, while the 1940–1946 epidemic coincides with Globe State of war II. There has been all-encompassing discussion of the role of colonial governance in the 1900–1920 sleeping sickness outbreak [11, 21, 27, 48–51]. The more recent T. b. rhodesiense epidemic in 1976–1990s coincided with political instability and civil state of war during and after the rule of Idi Amin [24]. Uganda's civil war influenced the transmission potential of sleeping sickness in a number of ways, including: breakup of veterinary and public wellness services (↑1/r) [52–54]; collapse of vector control (↑one thousand, ↑1/u); regrowth of bushy tsetse habitat in abased agricultural fields (↑yard) [nineteen, 52]; increasing displacement of human and beast populations into marginal or swampy areas where they are more likely to exist bitten by flies (↑α) [52]; In Uganda, internally displaced people (IDP) fled areas of intense disharmonize, particularly in the 'Luwero Triangle' region (Figure ane), an area in south-central Republic of uganda where much of the conflict and violence was concentrated [55, 56]. Refugees and IDPs returning home after the conflict faced increased risk due to the vegetation and new tsetse habitat that had grown during their absenteeism (↑m, ↑α). These processes direct affected a number of parameters in the sleeping sickness manual model (k, α, i/u, 1/r), increasing the transmission potential of the disease (R0). The R0 value increased via these processes over several years (resulting in a time lag between the peak in civil disharmonize and the outbreak acme) earlier exceeding the threshold required for an outbreak.
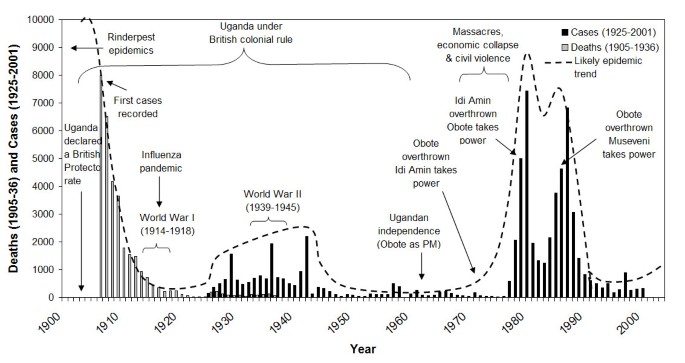
Sleeping sickness epidemics and major political events in Republic of uganda, 1905–2000. Cases from 1936 onwards include s-eastern Uganda merely. Sources: Sleeping sickness data 1905–36 deaths [62], 1925–36 cases [62], 1937–58 cases [63], 1960–71 cases (Unpublished study, 1992, Mbulamberi, D. B. The sleeping sickness situation in Uganda: by and nowadays. National Sleeping Sickness Control Program, Jinja, Uganda), 1972–75 cases [54], and 1976–2001 cases (Ministry of Health, Uganda); Political fourth dimension-series [56, 64, 65].
Conflict and sleeping sickness in sub-Saharan Africa
The trends and processes contributing to southward-eastern Uganda's sleeping sickness epidemic accept too been observed in other countries. Despite the epidemiological differences between T. b. rhodesiense and T. b. gambiense, the temporal occurrence of outbreaks or high incidence of both sub-species are associated with times of conflict. An association between land cover change and sleeping sickness similar to the procedure observed in south-eastern Uganda has been suggested in Republic of kenya; increased vegetation growth around homesteads and the resulting move of tsetse flies into peridomestic environments likely contributed to the sleeping sickness outbreaks in Kenya in 1965 [57]. Outbreaks in the 1990s in Sudan, equally in south-eastern Republic of uganda, accept been linked to abandonment of land, bush invasion, and increased run a risk of exposure for returning IDPs [52]. In north-western Uganda in the Due west Nile region, T. b. gambiense infection is believed to accept been introduced to the area by refugees returning from infected areas of Sudan following Uganda's ceremonious war [58].
Plummet of essential health services, and veterinary and vector control contribute consistently to increased disease risk through impacts on the transmission parameters of sleeping sickness (1000, 1/r, and 1/u). Reduced surveillance and handling directly bear upon the duration of infection in both humans and animal reservoirs of infection (↑i/r), while reduction of health and veterinary services increase the elapsing of infection in people and infected animals (↑i/r). Collapse of vector command can afflicted both tsetse numbers (↑thou) and the boilerplate lifespan of flies in the area (↑1/u). Surveillance and treatment are particularly important for the mitigation and control of T. b. gambiense. In Sudan, for example, absence of active case finding was a major factor contributing to the resurgence of sleeping sickness in the 1990s [18]. Sudanese health and affliction control infrastructure was essentially non-operable during two decades of ceremonious state of war in the 1970s-1990s [19]. In the DRC, negligible staff salaries, lack of motivation, poor road conditions, petrol shortages, and corruption were identified as key constraints to the efficacy of mobile surveillance teams, which failed to contain sleeping sickness incidence in the 1990s [19]. Limited or absent veterinary and vector control programs tin can be more important in T. b. rhodesiense areas, where livestock reservoirs can have considerable influence on transmission potential [21, 59]. Insecurity due to conflict further constrains the capacity of both national governments and external organizations to respond to outbreak situations. In Republic of angola, for case, peripheral mining regions were subject to agile insurgencies, resulting in high insecurity; this made implementation of sleeping sickness command activities logistically impossible [threescore]. Collapse of training schools for health intendance workers, nurses and laboratory technicians contribute to further turn down in sleeping sickness control initiatives (↑ 1/r). Abel et al. [sixty] note that in the 1990s, at least two Angolan diagnostic and treatment centres had to exist abased due to rebel attacks.
In many state of war-zone outbreaks, non-governmental organizations (NGO) and United Nations (United nations) agencies assume fractional or full responsibility for outbreak response [five, eighteen]. In Angola in the 1990s, for example, religious organizations played an of import role in intervention activities when high insecurity in the country express Un and international NGO intervention in remote areas [60]. While external humanitarian back up may contribute essential services, lack of harmonization and integration of activities between organizations is a constraint to consistent and continuous sleeping sickness control during conflict [18, 19]. In some cases, resurgence of sleeping sickness during periods of conflict has occurred in previous affliction hot spots, as in the case of Sudan [xviii]; in others, resurgence is occurring outside of traditional disease foci, as is currently occurring in sleeping sickness spread into central Uganda [24].
The range of specific processes by which conflict has contributed to sleeping sickness chance is summarized in Tabular array ane. This summary identifies the dominant impacts and vulnerabilities associated with conflict, their effects on the transmission determinants of sleeping sickness, and the resulting touch on on the R0 equation parameters of sleeping sickness. Four fundamental affect categories are identified, including: ane) Economic and global furnishings, two) Decline of health systems and services, iii) Forced migration and internal displacement of populations, and 4) Regional insecurity and restricted access for external humanitarian support.
The proportionate importance of the factors summarized in Table 1 will differ between rhodesiense and gambiense afflicted areas. Transmission during m ambiense outbreaks is usually dominated past a homo-fly-human wheel. Reduced handling of humans and decline of wellness systems will therefore have greater impact in gambiense afflicted regions. In these areas, decline of health services leads to increased elapsing of human infection (↑1/r in humans). Changes in livestock infection volition have greater impact in rhodesiense afflicted regions. In these areas, civil war leads to reduced cattle treatment, thus increasing the duration of cattle infection (↑i/r in cattle) and the reservoir of human-infectious parasites for transmission to the man population. In both gambiense and rhodesiense affected regions, reduced tsetse control increases vector populations (↑m, ↑1/u). Displacement of people and animals into marginal, bushy or swampy areas further promotes increased human, fly, and cattle contact (↑a).
Discussion
Conflict is a major determinant of sleeping sickness outbreaks in sub-Saharan Africa. Efforts to prevent and control sleeping sickness must identify and integrate noesis of the processes by which conflict affects disease take a chance. Prioritization of high-run a risk areas and targeted intervention can be optimized by consideration of disharmonize in sleeping sickness-affected countries. Sleeping sickness intervention in Africa is constrained by a range of factors and processes. Increased drug development is needed to identify and develop newer, safer drugs with more secure availability and supply to the African marketplace. The epidemic nature of sleeping sickness means that when cases decline, resources are often rapidly reallocated to other wellness priorities until the occurrence of another outbreak. Consistent and active surveillance is hard to rationalize during inter-outbreak periods in afflicted countries where resources are strained. Since late-phase sleeping sickness cases are expensive and difficult to treat, however, active surveillance, early treatment, and outbreak prevention can considerably reduce the brunt of disease. In countries recovering from contempo ceremonious state of war, rapid re-establishment of essential health services and active surveillance and treatment volition exist central to reducing sleeping sickness incidence. The focal nature of sleeping sickness means that resources tin be optimized in the short term past targeting outbreak locations and areas bordering countries with high incidence. In cases such as Uganda, where conflict is intermittent and regional, intervention is not extensively constrained by insecurity. Mobilization of national and international resource to support intervention in central Uganda is logistically feasible. As noted by Fèvre et al. [2], T. b. rhodesiense is theoretically not difficult to prevent, but can exist challenging to control one time established. In the case of T. b. rhodesiense in Republic of uganda, surveillance and control of livestock infection/movements can contribute to a decrease in both the animal reservoir of infection and spread of the parasite to new populations. Much of this support volition need to be targeted at policy and infrastructure development. Central Uganda is currently experiencing spread of disease and establishment of new T. b. rhodesiense foci; rapid intervention to curb these developments is needed to preclude increased burden of disease due to sleeping sickness in Uganda.
Conflict has played an of import role in contributing to the incidence and distribution of sleeping sickness in sub-Saharan Africa. Regrettably, the causes of sleeping sickness are also the primary constraints to eradication initiatives: "near past definition, [sleeping sickness] is a public health problem in places where a research infrastructure tin can hardly be" [[sixty] p. 147]. The campaign to eliminate the tsetse vector from the African continent [12, 14, 61] will face up enormous constraints due to continued conflict. Absence of appropriate administrative infrastructures for program implementation in afflicted countries represents the well-nigh acute challenge to such campaigns; this is true even for countries such as Republic of uganda where conflict is only intermittent or regional. In Uganda, authoritative chapters and intersectoral cooperation are important constraints to coordination of intervention activities [2]. While top-down, continent-wide eradication campaigns are ambitious and appealingly goal-oriented, progress to adjourn sleeping sickness is more than probable to come from wearisome evolution of national capacity, policy infrastructure, administrative integration and political stabilization in affected countries. In many cases, insecurity due to conflict has constrained international or external intervention and control. Local interventions, with localized infrastructure and rural deployment capacity, may be better placed to provide essential services during times of intense or widespread conflict. Donors and aid agencies should continue to support national, regional, and customs mitigation and intervention initiatives towards the common goal of reducing and eliminating sleeping sickness burden.
Prioritization of high-risk areas for sleeping sickness and trypanosomiasis command should explicitly integrate the occurrence of conflict and its impacts on transmission risk. The occurrence of disharmonize and presence of big number of internally displaced people tin be integrated into current risk maps in add-on to land comprehend, tsetse habitat, and livestock distributions. An understanding of areas where conflict may contribute to increased disease risk can guide prioritization of continent-broad as well as national mitigation programs.
Determination
Conflict is an of import determinant of sleeping sickness outbreaks in sub-Saharan Africa. In Uganda, the ii sub-species of sleeping sickness tin can be expected to merge in the absence of firsthand and targeted intervention in central districts; the presence of both diseases in one region will dramatically increase brunt of illness as well as the complexity and difficultly of subsequent command initiatives. Control and prevention of sleeping sickness by national and international regime should explicitly integrate consideration of conflict and its impacts into mapping and targeting of regions for priority intervention. Prevention and control campaigns should be assessed and evaluated against the ability of the initiative to address, mitigate, or alleviate the conflict-related drivers of illness chance. The occurrence of sleeping sickness in conflict-afflicted areas volition severely constrain the success and cost-benefit evaluations of continent-wide tsetse eradication campaigns. Prevention of sleeping sickness take a chance in affected sub-Saharan African countries requires increased international focus on development of administrative policy, chapters, integration, and infrastructure to implement localized control strategies.
Abbreviations
- HIV/AIDS:
-
Human Immuno-deficiency Virus/Acquired Immuno-deficiency Virus
- DRC:
-
Autonomous Democracy of the Congo
- WHO:
-
World Health Organization
- IDP:
-
Internally Displaced Persons
References
-
Berrang-Ford Fifty, Waltner-Toews D, Charron D, Odiit M, McDermott J, Smit B: Sleeping sickness in southeastern Republic of uganda: a systems approach. EcoHealth. 2005, two.
-
Fèvre EM, Picozzi Thou, Waiswa C, Odiit One thousand, Coleman PG, Welburn SC: A burgeoning epidemic of sleeping sickness in Uganda. The Lancet. 2005, 366: 745-747. 10.1016/S0140-6736(05)67179-6.
-
Odiit Yard, Coleman PG, Liu WC, McDermott JJ, Fèvre EM, Welburn SC, Woolhouse EMEJ: Quantifying the level of under-detection of Trypanosoma brucei rhodesiense sleeping sickness cases. Tropical Medicine and International Health. 2005, x: 840-849. 10.1111/j.1365-3156.2005.01470.x.
-
Moore A, Richer M, Enrile M, Losio Due east, Roberts J, Levy D: Resurgence of sleeping sickness in Tambura Canton, Sudan. American Periodical of Tropical Medicine and Hygiene. 1999, 61: 315-318.
-
Stanghellini A, Gampo S, Sicard JM: The function of environmental factors in the present resurgence of human African trypanosomiasis [Role des facteurs environnementaux dans la recrudescence actuelle de la trypanosomiase humaine africain]. Bulletin de la Societe de Pathologie exotique. 1994, 87: 303-306.
-
Jordan AM: Trypanosomiasis command and country use in Africa. Outlook on Agronomics. 1979, x: 123-129.
-
Hashemite kingdom of jordan AM: Trypanosomiasis Control and African Rural Evolution. 1986, Harlow, Longman
-
Leak SGA: Tsetse Biology and Ecology: Their Role in the Epidemiology and Command of Trypanosomosis. 1999, Wallingford, United kingdom, CABI Publishing in clan with the International Livestock Enquiry Institute, Nairobi, Kenya, 568.
-
Mbulamberi DB: Recent advances in the diagnosis and handling of sleeping sickness. Postgraduate Doctor Africa. 1994, xvi: 16-19.
-
Odiit M, Shaw A, Welburn SC, Fevre EM, Coleman PG, McDermott JJ: Assessing the patterns of health-seeking behaviour and awareness amidst sleeping-sickness patients in eastern Uganda. Annals of Tropical Medicine and Parasitology. 2004, 98: 339-348. 10.1179/000349804225003389.
-
Ford J: The Role of the Trypanosomiases in African Ecology. 1971, Oxford, Clarendon Press, approx. 550 pp..
-
Legros D, Ollivier Thousand, Gastellu-Etchegorry M, Paquet C, Burri C, Jannin J, Büscher P: Treatment of human being African trypanosomiasis - present situation and needs for research and development. Lancet Infectious Diseases. 2002, two: 437-440. 10.1016/S1473-3099(02)00321-3.
-
Dumas Chiliad, Bouteille B: Treatment of man African trypanosomiasis. Message of the World Health Organisation. 2000, 78: 1474.
-
Maurice J: Continent-wide assault launched on African trypanosomiasis. Bulletin of the World Health Organisation. 2001, 79: 1087.
-
Pécoul B, Chirac P, Trouiller P, Pinel J: Access to essential drugs in poor countries. A lost battle?. Periodical of the American Medical Association. 2006, 281: 361-367. 10.1001/jama.281.four.361.
-
Van Nieuwenhove Due south: Gambiense sleeping sickness: re-emerging and soon untreatable?. Message of the World Health Organisation. 2000, 78: 1283.
-
Yamey G: The world's most neglected diseases: ignored past the pharmaceutical industry and by public-private partnerships. British Medical Periodical. 2002, 325: 176-177. 10.1136/bmj.325.7357.176.
-
Moore A, Richer Chiliad: Re-emergence of epidemic sleeping sickness in southern Sudan. Tropical Medicine and International Wellness. 2001, 6: 342-347. 10.1046/j.1365-3156.2001.00714.x.
-
Smith DH, Pépin J, Stich AHR: Human African trypanosomiasis: an emerging pubic wellness crisis. British Medical Bulletin. 1998, 54.
-
Musere J: African Sleeping Sickness: Political Ecology, Colonialism and Command in Republic of uganda. 1990, New York, Edwin Mellen
-
Fèvre EM, Coleman PG, Welburn SC, Maudlin I: Reanalyzing the 1900-1920 sleeping sickness epidemic in Uganda. Emerging Communicable diseases. 2004, 10: Available from: http:www.cdc.gov/ncidod/EID/vol10no4/02-0626.htm.
-
de Raadt P: Towards year 2000: what prospects for human being African trypanosomiasis? [ Horizon 2000: quelle perspective cascade la trypanosomiase africaine?]. Bulletin de la Societe de Pathologie exotique. 1994, 87: 301-302.
-
World Health System: Control and Surveillance of African Trypanosoiasis. Written report of a WHO Expert Committee on Sleeping Sickness. Technical Report Series. 1998, Geneva, WHO
-
Berrang-Ford L, Berke O, Abdelrahman Fifty, Waltner-Toews D, McDermott J: Spatial analysis of sleeping sickness in due south-eastern Uganda, 1970-2003. Emerging Infectious Affliction. 2006, 12: 813-820.
-
Hutchinson OC, Fèvre EM, Carrington K, Welburn SC: Lessons learned from the emergence of a new Trypanosoma brucei rhodesiense sleeping sickness focus in Republic of uganda. The Lancet Infectious Diseases. 2003, 3: 42-45. x.1016/S1473-3099(03)00488-2.
-
Welburn SC, Odiit Thou: Recent developments in human being African trypanosomiasis. Electric current Opinion in Infectious Diseases. 2002, 15: 477-484.
-
Hide Chiliad, Tait A, Maudlin I, Welburn SC: The origins, dynamics and generation of trypanosoma brucei rhodesiense epidemics in East Africa. Parasitology Today. 1996, 12: 50-55. 10.1016/0169-4758(96)80654-5.
-
World Health System: Study on African trypanosomiasis (sleeping sickness), Report of the Scientific Working Group Meeting on African Trypanosomiasis, 4-8 June 2001. TDR/SWG/01. 2001, Geneva, Special Plan for Research and Grooming in Tropical Diseases, Available on-line at: http://whqlibdoc.who.int/hq/2003/TDR_SWG_01.pdf
-
Khonde Northward, Pépin J, Niyonsenga T, Milord F, Wals PD: Epidemiological evidence for amnesty following Trypanosoma brucei gambiense sleeping sickness. Transactions of the Royal Lodge of Tropical Medicine and Hygiene. 1995, 89: 607-611. x.1016/0035-9203(95)90408-5.
-
Edeghere H, Olise PO, Olatunde DS: Human African trypanosomiasis (sleeping sickness): new endemic foci in Bendel Land, Nigeria. Tropical Medicine and Parasitology. 1989, xl: xvi-xx.
-
Salama P, Speigel P, Talley L, Waldman R: Lessons learned from circuitous emergencies over the past decade. The Lancet. 2004, 364: 1801-1813. 10.1016/S0140-6736(04)17405-9.
-
Connolly M, Gayer M, Ryan M, Salama P, Spiegel P, Heymann D: Communicable diseases in complex emergencies: impacts and challenges. The Lancet. 2004, 364: 1974-1983. ten.1016/S0140-6736(04)17481-3.
-
McMichael T: Man Frontiers, Environments and Disease. 2001, Cambridge, Cambridge University Printing
-
McNeill WH: Plagues and People. 1977, New York, Doubleday
-
Diamond J: Guns, Germs, and Steel: the Fates of Homo Societies. 1997, New York, Westward. W. Norton
-
Garfield RM: Epidemiologic analysis of warfare: a historical review. Periodical of the American Medical Association. 1991, 266: 688-692. 10.1001/jama.266.five.688.
-
Coghlan B, Brennan R, Ngoy P, Dofara D, Otto B, Clements M, Stewart T: Mortality in the Democratic Republic of Congo: a nationwide survey. Lancet. 2006, 367: 44-51. 10.1016/S0140-6736(06)67923-3.
-
Global Polio Eradication Initiative: Available on-line at: www.polioeradication.org. 2006
-
Tangermann RH, Hull HF, Jafari H, Nkowane B, Everts H, Aylward RB: Eradication of poliomyelitis in countries affected past conflict. Bulletin of the World Health Organization. 2000, 78: 330-338.
-
Platt AE: Infecting Ourselves: How Environmental and Social Disruptions Trigger Affliction. 1996, Washington, D. C., Worldwatch Institute
-
Lawrence JA, Foggin CM, Norval RA: The furnishings of war on the control of diseases of livestock in Rhodesia (Republic of zimbabwe). Veterinarian Tape. 1980, 107: 82-85.
-
Briand S, Khalifa H, Peter C, Khatib O, Bolay F, Woodruff B, Anderson Chiliad, Mintz E: Cholera epidemic afterwards increased ceremonious disharmonize - Monrovia, Liberia, June-September 2003. Morbidity and Bloodshed Weekly Report. 2003, 52: 1093-1095.
-
Smallman-Raynor MR, Cliff Advertisement: Civil war and the spread of AIDS in Central Africa. Epidemiology and Infection. 1991, 107: 69-80.
-
Rogers DJ: A full general model for the African trypanosomiases. Parasitology. 1988, 97: 193-212.
-
McDermott JJ, Coleman PG: Comparing apples and oranges - model-based assessment of different tsetse-transmitted trypanosomosis command strategies. International Periodical for Parasitology. 2001, 31: 603-609. ten.1016/S0020-7519(01)00148-5.
-
Rogers DJ, Williams BG: Monitoring trypanosomiasis in infinite and time. Parasitology. 1993, 106: S77-S92.
-
Carpenter GDH: A naturalist on Lake Victoria: with an Account of Sleeping Sickness and the Tsetse Fly. 1920, London, T. Fisher Unwin Ltd.
-
Lyons Thou: African sleeping sickness: an historical review. International Journal of STD and AIDS. 1991, two: 20-25.
-
Worboys M: The comparative history of sleeping sickness in East and Cardinal Africa, 1900-1914. History of Science. 1994, 32: 89-102.
-
Hide G: History of sleeping sickness in East Africa. Clinical Microbiology Reviews. 1999, 12: 112-125.
-
Mbulamberi DB: Possible causes leading to an epidemic outbreak of sleeping sickness: facts and hypotheses. Annales de la Société Belge de Médecine Tropicale. 1989, 69: 173-179.
-
Abaru DE: Sleeping sickness in Busoga, Uganda, 1976-1983. Tropical Medicine and Parasitology. 1985, 36: 72-76.
-
Okiria R: The prevalence of human trypanosomiasis in Uganda, 1970 to 1983. Eastward African Medical Journal. 1985, 62: 813-816.
-
Museveni YK: Sowing the Mustard Seed: the Struggle for Freedom and Republic in Uganda. 1997, London, MacMillan
-
Kasozi ABK: The Social Origins of Violence in Uganda. 1994, Kampala, Uganda, Fountain Publishers Ltd.
-
Okoth JO: Peri-domestic convenance sites of Glossina fuscipes fuscipes Newst. in Busoga, Uganda and epidemiological implications for trypanosomiasis. Acta Tropica. 1986, 43: 283-286.
-
Mbulamberi DB: The sleeping sickness state of affairs in Uganda: past and present (unpublished report), National Sleeping Sickness Control Program, Jinja, Uganda. 1992
-
Fèvre EM, Coleman PG, Odiit M, Magona JW, Welburn SC, Woolhouse MEJ: The origins of a new Trypanosoma brucei rhodesiense sleeping sickness outbreak in eastern Uganda. The Lancet. 2001, 358: 625-628. x.1016/S0140-6736(01)05778-6.
-
Abel PM, Kiala G, Loa Five, Behrend M, Musolf J, Fleischmann H, Theophile J, Krishna S, Stich A: Retaking sleeping sickness control in Angola. Tropical Medicine and International Health. 2004, 9.
-
Stich A, Barrett MP, Krishna Due south: Waking up to sleeping sickness. Trends in Parasitology. 2003, nineteen: 195-197. 10.1016/S1471-4922(03)00055-2.
-
Republic of uganda Protectorate: Almanac Medical and Germ-free Records. 1908, Government Printer, Entebbe, Located at The National Athenaeum, Uganda, Open Shelf Library. Assembled by Lea Berrang, 2002.
-
Soff HG: A History of Sleeping Sickness in Uganda: Administrative Response 1900-1970. Modern History. 1971, Syracuse, New York, Syracuse University, approx. 280 pp..
-
Adu Boahen A: Vii Africa Under Colonial Domination 1880-1935. Full general History of Africa, Abridged Edition. 1990, Paris, UNESCO
-
Kyemba H: A State of Blood. 1977, Kampala, Uganda, Fountain Publishers Ltd.
Acknowledgements
Research was supported by stipend funding from the National Science and Engineering Research Council of Canada (NSERC). Special thanks to Dr. Mbulamberi, Ministry of Wellness, Republic of uganda; Dr. Maiso, Earth Wellness Organization, Kampala; Dr. Oddit, Kampala; Lubowa Abdelrahman, Kampala; Dr. James Ford, Department of Geography, University of Guelph; Drs. David Waltner-Toews and Olaf Berke, Section of Population Medicine, Academy of Guelph; Dr. John McDermott, International Livestock Enquiry Institute, Nairobi, Kenya; and the belatedly Dr. Gitau for contributions towards conception, design, and/or manuscript revision.
Author information
Affiliations
Corresponding author
Boosted information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
LBF conceived, designed, researched, and prepared the manuscript.
Authors' original submitted files for images
Rights and permissions
This article is published nether license to BioMed Cardinal Ltd. This is an Open Access article distributed nether the terms of the Artistic Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted utilise, distribution, and reproduction in any medium, provided the original piece of work is properly cited.
Reprints and Permissions
About this commodity
Cite this article
Berrang Ford, Fifty. Civil disharmonize and sleeping sickness in Africa in general and Uganda in particular. Confl Health 1, half dozen (2007). https://doi.org/x.1186/1752-1505-i-six
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/1752-1505-one-6
Keywords
- Trypanosomiasis
- Civil Conflict
- Eradication Campaign
- Affected Country
- Internally Displace People
Source: https://conflictandhealth.biomedcentral.com/articles/10.1186/1752-1505-1-6
0 Response to "East African Sleeping Sickness Affect on the Angel Again War"
Enregistrer un commentaire